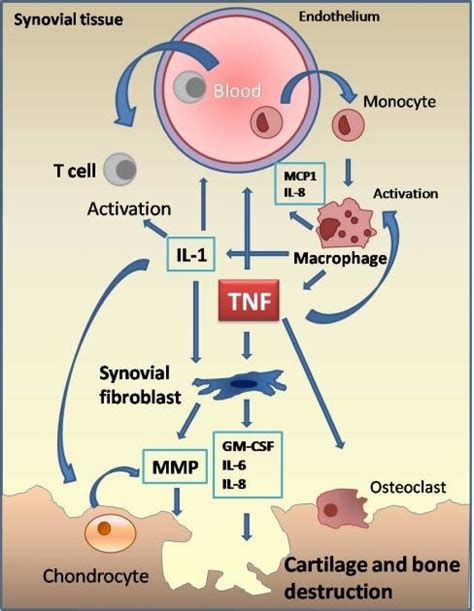
Pathogenesis Rheumatoid Arthritis
The pathogenesis of rheumatoid arthritis is complex.
Click on the active elements to find your solution
TNF a
Tumor necrosis factor-alpha (TNF-alpha) is a protein that plays a key role in the immune system's response to infections and injuries. It is produced by several types of immune cells, including macrophages, which are involved in the inflammation that occurs in rheumatoid arthritis (RA).
In RA, the immune system mistakenly attacks the lining of the joints, causing inflammation and damage. TNF-alpha is one of several pro-inflammatory cytokines that are overproduced in RA, contributing to the inflammation and damage to the joints. Excessive levels of TNF-alpha can activate other immune cells,
By targeting TNF-alpha, it is possible to reduce inflammation and prevent further joint damage in people with RA.
Button
Interleukin-1
Interleukin-1 (IL-1) is a pro-inflammatory cytokine associated with joint inflammation in rheumatoid arthritis (RA). It is produced by synovial cells, macrophages, endothelial cells, and fibroblasts in response to an immune stimulus.
High levels of IL-1 can cause joint swelling, pain, and stiffness. It also stimulates the production of other pro-inflammatory cytokines such as tumor necrosis factor alpha (TNFα) and interferon gamma (IFNγ), which worsen the inflammation and cause joint destruction.
In addition, IL-1 affects chondrocyte proliferation, ECM synthesis, and cell death. This can lead to cartilage degradation in conditions like osteoarthritis (OA) and RA. Understanding how IL-1 contributes to RA could help guide development of effective treatments for this difficult condition.
Button
GM-CSF
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a pro-inflammatory cytokine associated with joint inflammation in rheumatoid arthritis (RA). It is produced by synovial cells, macrophages, endothelial cells, and fibroblasts in response to an immune stimulus.
GM-CSF can induce the production of other pro-inflammatory cytokines such as tumor necrosis factor alpha (TNFα) and interferon gamma (IFNγ), which worsens the inflammation and causes joint destruction. It also modulates the differentiation, maturation and function of leukocytes as well as stimulates chondrocyte proliferation, ECM synthesis, and cell death. This can lead to cartilage degradation in conditions like osteoarthritis (OA) and RA.
Button
Synovial Fibroblasts
Synovial Fibroblasts (SF) are the major cell type found in the joint lining of rheumatoid arthritis (RA). They play an important role in inflammation, tissue destruction, and remodelling. SFs produce a variety of proinflammatory cytokines including interleukin-1 (IL-1) and tumor necrosis factor alpha (TNFα) which contribute to joint swelling, pain and stiffness.
In addition, SFs secrete proteases that can break down cartilage and bone matrix proteins, leading to cartilage degradation in RA. They also release chemokines that can activate leukocytes and promote angiogenesis which further contributes to tissue damage.
Button
Osteoclasts
Osteoclasts are specialized bone-resorbing cells involved in the pathogenesis of rheumatoid arthritis (RA). These cells are formed through a process of differentiation from monocytes and macrophages, which leads to an increase in their production, activation, and survival.
Osteoclasts release pro-inflammatory cytokines such as IL-1β and TNFα that contribute to joint inflammation in RA. They also secrete proteases and other enzymes that degrade cartilage matrix proteins, leading to cartilage destruction.
In addition, osteoclasts can play a role in bone remodelling associated with RA by increasing bone turnover rate. Understanding the role of these cells can help guide the development of new treatments for this debilitating condition.
Button
Macrophages
Macrophages play an important role in the pathogenesis of rheumatoid arthritis (RA). These cells are attracted to joint synovial fluid, where they secrete pro-inflammatory cytokines such as IL-1β and TNFα, which contribute to joint inflammation. They also produce matrix metalloproteases and other enzymes which degrade cartilage matrix proteins, leading to cartilage destruction.
Macrophages can also promote angiogenesis by releasing chemokines that recruit endothelial cells and stimulate blood vessel formation. They may be involved in bone remodelling associated with RA due to their ability to phagocytose dying cells and release peptides that stimulate osteoclasts.
Button
T cells
T cells are key players in the pathogenesis of rheumatoid arthritis (RA). They play an important role in driving joint inflammation by secreting cytokines such as IFN-γ, IL-17 and TNFα.
T cells can also mediate tissue destruction in RA by inducing macrophages to produce proteases and other enzymes that degrade cartilage matrix proteins. In addition, they may be involved in promoting angiogenesis and bone remodelling, which can exacerbate inflammatory symptoms.
Button
Monocytes
Monocytes are important cells involved in the pathogenesis of rheumatoid arthritis (RA). They are recruited to joint synovial fluid and secrete pro-inflammatory cytokines such as IL-1β and TNFα, which contribute to joint inflammation.
Monocytes can also produce matrix metalloproteases and other enzymes that degrade cartilage matrix proteins, leading to tissue destruction. In addition, they may promote angiogenesis by releasing chemokines that recruit endothelial cells and stimulate blood vessel formation.
Button
Interleukin 8
Interleukin 8 (IL-8) is a pro-inflammatory chemokine that plays a key role in the pathogenesis of rheumatoid arthritis (RA). It is upregulated in RA patients and activates monocytes, neutrophils, and T cells to initiate an immune response.
IL-8 also promotes the release of proteases and other enzymes that degrade cartilage matrix proteins, leading to joint destruction. In addition, it may be involved in promoting angiogenesis, which can exacerbate inflammation.
Button
MCP-1
Monocyte Chemoattractant Protein-1 (MCP-1) is an important cytokine involved in rheumatoid arthritis (RA). It is released by monocytes, macrophages and other immune cells, and acts as a chemoattractant for neutrophils. Once recruited to the site, these cells can cause inflammation and tissue damage.
MCP-1 also promotes angiogenesis, stimulating the formation of new blood vessels that can enhance the inflammatory process. In addition, it may increase cartilage degrading enzymes, leading to joint destruction.
Button
Synovial tissue
Synovial tissue is a specialized connective tissue that lines synovial joints, which are the most common type of joint in the human body. Synovial joints are characterized by the presence of a synovial cavity that is filled with synovial fluid, a clear, viscous liquid that lubricates the joint and provides nutrients to the cartilage.
Button
The endothelium
The endothelium is a layer of cells that lines the inner surface of blood vessels and organs. It serves as a barrier between the body's tissues and the bloodstream, allowing for the regulation of substances passing in and out of the circulatory system
The endothelium plays an important role in the pathogenesis of rheumatoid arthritis (RA). It is upregulated in RA patients and releases chemokines and adhesion molecules that recruit immune cells to the site, promoting inflammation.
The endothelium can also be involved in angiogenesis, which can stimulate the inflammatory process. In addition, it may increase cartilage degrading enzymes, leading to joint destruction.
Button
MMP
Matrix metalloproteinases (MMPs) are a group of enzymes that degrade the extracellular matrix (ECM), a complex network of proteins and other molecules that provides structural support to cells and tissues. MMPs are involved in several physiological processes, including tissue remodeling, wound healing, and angiogenesis.
In joints, MMPs play a role in the physiological turnover of the ECM and contribute to the maintenance of joint homeostasis. However, in inflammatory joint diseases like rheumatoid arthritis (RA), an imbalance between MMPs and their inhibitors can lead to excessive ECM degradation and joint damage.
Button
Chondrocytes
Chondrocytes are specialized cells that produce and maintain the extracellular matrix (ECM) of cartilage. Cartilage is a flexible connective tissue that provides structural support to several parts of the body, including joints, the rib cage, and the ears.
Chondrocytes are found in small spaces called lacunae within the ECM of cartilage. They are responsible for synthesizing and organizing the components of the cartilage ECM, including collagen, proteoglycans, and glycosaminoglycans.
Button
IL-6
In rheumatoid arthritis (RA), interleukin-6 (IL-6) is a pro-inflammatory cytokine that plays an important role in inflammation, joint destruction, and immune regulation.
In the synovium, IL-6 can activate B cells to produce autoantibodies directed against citrullinated proteins. This helps initiate the inflammatory cascade. In addition, IL-6 can stimulate the production and differentiation of Th17 cells and regulatory T cells (Tregs). The activation of Th17 cells results in increased levels of other pro-inflammatory cytokines such as interferon gamma (IFNγ) and tumor necrosis factor alpha (TNFα). These cytokines contribute to joint inflammation and destruction.
Button
IL- 8
Interleukin-8 (IL-8) is a pro-inflammatory cytokine produced by various cell types in response to inflammation and tissue damage. In rheumatoid arthritis (RA), IL-8 is upregulated and plays an important role in the inflammatory process.
IL-8 can regulate several cellular functions, such as inducing migration of monocytes, neutrophils, and lymphocytes into the joints of patients with RA. It also stimulates the production of other pro-inflammatory cytokines such as tumor necrosis factor alpha (TNFα) and interferon gamma (IFNγ), which worsen the inflammation and cause joint destruction.
In addition, IL-8 affects chondrocyte proliferation, ECM synthesis, and cell death. This can lead to cartilage degradation in conditions like osteoarthritis (OA) and RA. The understanding of how IL-8 contributes to RA could help guides development of effective treatments for this difficult condition.
Button
